Cell & Developmental Biology of Xenopus:
Gene Discovery & Disease
March 29 - April 11, 2017
Application Deadline: January 31, 2017
Instructors:
Mustafa Khokha, Yale University
Karen Liu, King's College London, UK
Xenopus is remarkable for modeling human diseases including birth defects, cancer, and stem cell biology. Xenopus has and continues to make a major impact in our understanding of cell and developmental biology.
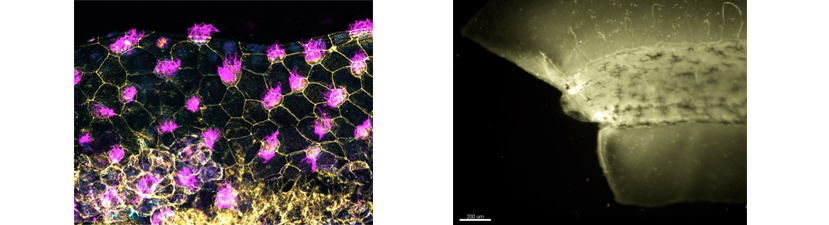
Students are encouraged to target genes of interest using CRISPR technology and then analyze phenotypes using the diverse array of assays available in Xenopus. Specifically, techniques covered include microinjection, and various molecular manipulations including, CRISPR knockouts, morpholino based depletions, transgenics, and mRNA overexpression. In addition, students can combine these techniques with explant and transplant methods to simplify or test tissue level interactions. To visualize subcellular and intercellular activities, we will introduce a variety of imaging methods including time-lapse, fluorescent and confocal microscopy. Additional methods include mRNA in situ hybridization and protein immunohistochemistry as well as basic bioinformatic techniques for gene comparison and functional analysis. Biochemical approaches such as proteomics and mass spectrometry will also be discussed.
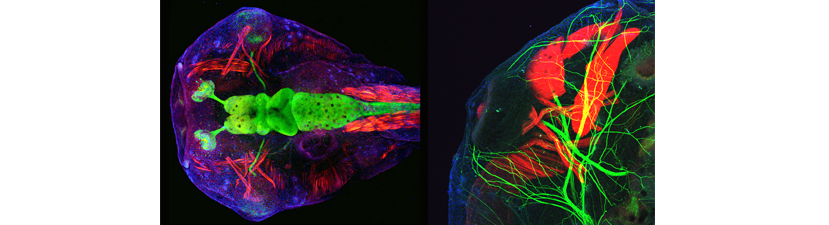
This course is designed for those new to the Xenopus field, as well as for more advanced students who are interested in emerging technologies. We encourage students to bring their own genes of interest and will tailor aspects of the course to enable them to initiate studies on their specific projects.
Confirmed 2017 Lecturers:
Ira Blitz, University of California, Irvine
Sang-Wook Cha, Cincinnati Children's Hospital Medical Center
Frank Conlon, University of North Carolina
Lance Davidson, University of Pittsburgh
Rebecca Heald, University of California, Berkeley
Raymond Keller, University of Virginia
Roberto Mayor, University College London, UK
Ann Miller, University of Michigan
Peter Nemes, George Washington University
Benjamin Steventon, University of Cambridge, UK
Gerald Thomsen, Stony Brook University
John Wallingford, University of Texas at Austin
Andrea Wills, University of Washington
10fps of Xenopus GRP cilia
10 frames per second imaging of Xenopus GRP cilia. GFP labeled cilia, RFP membrane marker. Courtesy of Melanie Tingler, Shiaulou Yuan, and Mustafa Khokha. Cold Spring Harbor Xenopus Course, Yale University. Movie generated on Bruker Opterra II confocal microscope.
125 fps blood flow
125 frames per second imaging of Xenopus red blood cells in gills. Playback at 5x slow motion. Video courtesy of Vaughn Colleluori and Mustafa Khokha, Cold Spring Harbor Xenopus Course,Yale University. Movie generated on Bruker Opterra II confocal microscope.
CLAMP GFP and RFP
CLAMP GFP (green) marks tips of cilia and membrane RFP (red) marks cilia axoneme. Two color images collected at 20 fps. CSHL 2015 Xenopus course. Movie generated on Bruker Opterra II confocal microscope.
Support & Stipends
Major support provided by the National Institute of Child Health and Human Development
Stipends are available to offset tuition costs as follows:


US applicants (National Institute of Child Health and Human Development )
Interdisciplinary Fellowships (transitioning from outside biology) & Scholarships (transitioning from other biological disciplines) (Helmsley Charitable Trust)
International applicants (Howard Hughes Medical Institute)
Please indicate your eligibility for funding in your stipend request submitted when you apply to the course. Stipend requests do not affect selection decisions made by the instructors.
Cost (including board and lodging): $3,810
This button links to a short form which confirms your interest in the course. No fees are due until you have completed the full application process and are accepted into the course.
Students accepted into the course should plan to arrive by early evening on March 28 and plan to depart after lunch on April 11.